QUICKpress: Capsule Information
Choice of sample container is crucial to asuccessful synthesis. It should be chemically inert, and provide a barrier toexchange of material between the sample and its surroundings. Containers can besealed or not depending on the sample. For instance, for oxide ceramics notsubject to redox reactions and containing low vapor pressure components, asample container is not needed so the graphite furnace can be used withgraphite disks placed at each end of the sample. This configuration maximizesthe sample volume. Alternatively graphite or boron nitride crucibles and lidscan be used, but these are permeable to fluids, so volatiles such as CO2 or N2will be at least partially lost from the sample. Sealed Containers prevent gainor loss of all volatiles except hydrogen.
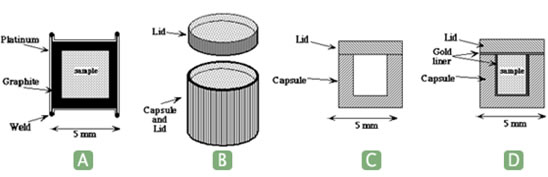
The most common sealed capsule is made ofthin platinum, gold, or palladium-silver alloy tubing with end caps of the samematerial welded together using an electric arc (Fig. 1a and b). Thecompatibility of the metal capsule with the sample must be carefully considered.Many transition metals are very soluble in platinum, causing their oxides todisproportionate, for example ferrous iron dissolves in platinum by thereaction 3 FeO = Fe(Pt) + Fe2O3. Reactions like this change both the bulkcomposition and oxidation state of the sample.
Sealed containers can also be fabricatedinto thick-walled crucibles with flat lids (Fig. 1 c). This works well when itis desired to saturate the system in a metal. For instance Ti metal capsuleswere used to investigate a portion of the system Ti - C - Si by Sambasivan andPetuskey (1992). A variation is to line the capsule with platinum or gold (Fig.1d). Alternatively, thick-walled silver capsules can be fabricated from rodstock.
Hydrogen is a nemesis in all high-pressureexperiments because of its very high permeability in metals, the difficulty ofremoving all water from furnace assemblies, and the reaction between water andthe graphite furnace to produce hydrogen. Hydrogen will diffuse either in orout of the sample container depending on the gradient in hydrogen chemicalpotential. This can be used to advantage to partially dehydrogenate samples byplacing a hydrogen sink (getter) such as Fe2O3 outside the sample container. Itcan also be a serious disadvantage if hydrogen diffuses into the capsule andreduces cations in high oxidation states. Oxygen activity can be controlled by,for example, the coexistence of a metal and its oxide. For instance, silvermetal together with Ag2O fixes oxygen activity at very high values. Other techniquesto fix oxygen activity in high pressure runs are summarized by Holloway andWood (1988).








